abbott point of care covid test
Our products provide highly accurate test results during the patient consultation using only a tiny fingerstick or urine sample. Solutions for respiratory diagnosis Abbott has rapid point-of-care solutions to support your COVID-19 and influenza testing needs ID NOW COVID-19 The ID NOW COVID-19 assay is now available for use on the ID NOW platform under US.
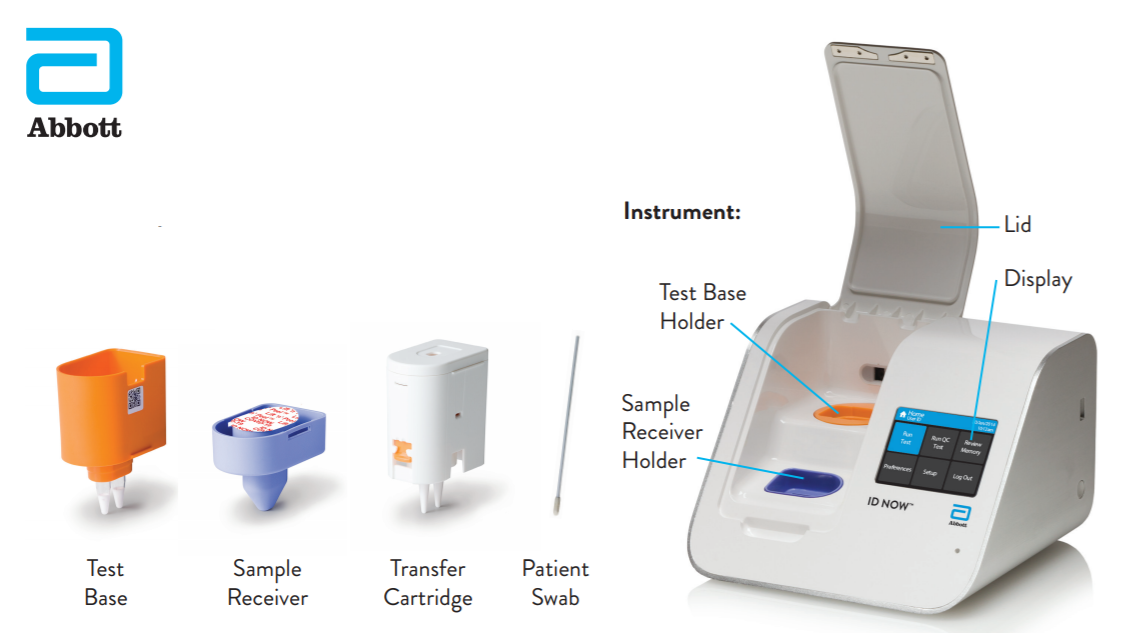
Instant Results From Abbotts Covid 19
Who can use Abbott ID NOW for point of care PCR.

. According to Abbott the rapid test which runs on the ID NOW platform is an. Food and Drug Administration Emergency Use Authorization EUA. NAVICA displays results from the 15-minute Abbott BinaxNOW COVID-19 Ag Card rapid antigen test to help individuals make informed decisions.
The value of point of care testing for cardiovascular risk and kidney disease management in diabetes and non-diabetes patients. A CLIA-certified laboratory or testing site must report all positive SARS-CoV-2 diagnostic and screening test results to the person who was tested or that persons healthcare provider. It is used on our ID NOW platform.
The BinaxNOW COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests. Abbott BinaxNOW COVID-19 Antigen Ag Card A self-contained antigen test that uses a card and does not require a separate analyzer device. The new access to POC PCR testing through the HCW Front of the Line Program will provide teams with additional capacity to support the fight against COVID-19.
In this prospective arm of the study the specificity of the Abbott ID NOW test using the kits dry swab was 999 when tested on 1043 prospectively collected paired swabs 95 CI 9947100. As a leader in diagnostic testing we have a unique responsibility to contribute our expertise to help fight the COVID-19 pandemic. Food and Drug Administration FDA for the fastest available molecular point-of-care test for the detection of novel coronavirus COVID-19 delivering positive results in as little as five minutes and negative results in 13 minutes.
ID NOW is an FDA approved CLIA-waived instrument which means that. To help provide the critical diagnostic information needed Abbott is currently providing and. CLIA-certified laboratories or testing sites are no longer required to report negative results for non-NAAT.
Reporting Requirements for Rapid Testing in Point-of-Care Settings. THE SCIENCE ID NOW PERFORMANCE FROM RESEARCHERS IN THE FIELD Reliable test results depend on many factors conformity to test design. Mar 28 2020 By Mark Terry Abbott s new point-of-care test for the novel coronavirus that causes COVID-19 was approved by the US.
The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that delivers results within minutes allowing healthcare professionals to. Food and Drug Administration FDA under Emergency Use Authorization EUA. The COVID-19 pandemic is affecting all of us around the world.
Abbotts Point-of-Care COVID-19 Test Detects Coronavirus in as Little as 5 Minutes Published. What makes this test so different is where it can be used. The revolutionary NAVICA app helps people navigate daily life in a new normal.
BinaxNOW COVID-19 Ag Card has received US. The TMBinaxNOW COVID-19 Ag Card is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS. The Abbott PanBio TM COVID-19 Ag point-of-care test was performed alongside RT-PCR.
Abbott ID NOW POC PCR testing creates faster access to COVID-19 testing and immediate access to test results for health-care workers and their families. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that delivers results within minutes allowing healthcare professionals to make clinical decisions during a patient visit. This study recruited participants presenting for COVID-19 testing at three Melbourne metropolitan hospitals during a period of low COVID-19 prevalence.
In addition participants with COVID-19 notified to the Victorian Government were invited to provide additional swabs to. Fifty-five participants were asymptomatic two had previously confirmed COVID-19 and 986 participants were symptomatic with suspected COVID-19 symptoms. Abbott has received emergency use authorization EUA from the US.
This document provides a step-by-guide to get started with Point of Care POC COVID-19 antigen testing. Food and Drug Administration FDA for the ID NOW COVID-19 test in March 2020. A health care worker takes a sample from a driver at a drive-thru COVID testing tent set up at Saratoga Hospital Wednesday April 27 2022 in Saratoga Springs NYLori Van BurenTimes Union Saratoga County will hold another free COVID-19 vaccine clinic Friday after.
Food and Drug Administration FDA for the fastest available molecular point-of-care test for the detection of novel coronavirus COVID-19 delivering positive results in as little as five minutes and negative results in 13 minutes. April 28 2022Updated. 03302020 Abbott launches rapid point-of-care COVID-19 test Sandra Levy Senior Editor The Food and Drug Administration on Friday issued Emergency Use Authorization for Abbotts molecular.
Abbott is putting its resources towards helping you navigate this crisis. Abbott received emergency use authorization EUA from the US. Point-of-care tests are critical to help fight the novel coronavirus pandemic.
Food and Drug Administration Emergency Use Authorization EUA. Currently the Virginia Department of Health VDH offers one type of prescription antigen test. April 28 2022 850 pm.
COVID-19 test Abbott Laboratories Abbott Park Illinois United States is said to be the most sensitive and specific PoCT on the global market with a sensitivity of 804 and Conclusion specificity of 959 with rRT-PCR as the standard1415 A study There was very poor IgM detection by most of the PoCT kits conducted to assess the quality. Chronic disease management in the post covid era. REDUCING RISK BY DIAGNOSING WITH RELIABLE RAPID TEST ID NOW delivers results in minutes where theyre needed most during COVID-19.
Abbott has received emergency use authorization EUA from the US. The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less. The Afinion 2 System is a.

Abbot Siapkan Alat Rapid Test Covid Khusus Di Indonesia Bisa Untuk Anak Dan Dewasa Lifestyle Bisnis Com

Abbott Id Now Covid 19 Detection Test System Us

As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News
/cdn.vox-cdn.com/uploads/chorus_asset/file/19856029/IDNOW_INACTION3_macro_300dpi_1200x628.jpg)
A New Covid 19 Test Can Return Results In 5 Minutes The Verge
/cdn.vox-cdn.com/uploads/chorus_asset/file/19856029/IDNOW_INACTION3_macro_300dpi_1200x628.jpg)
A New Covid 19 Test Can Return Results In 5 Minutes The Verge

Laboratory Id Now Abbott Point Of Care Test Pcr Mobile Device Autodoc

Steps To Use Id Now Effectively Abbott Newsroom

14 000 Rapid Covid 19 Testing Kits Coming To Grey Bruce Ctv News

Indonesia Go Id Cartridge Nya Isi Ulang Diagnosisnya Lima Menit
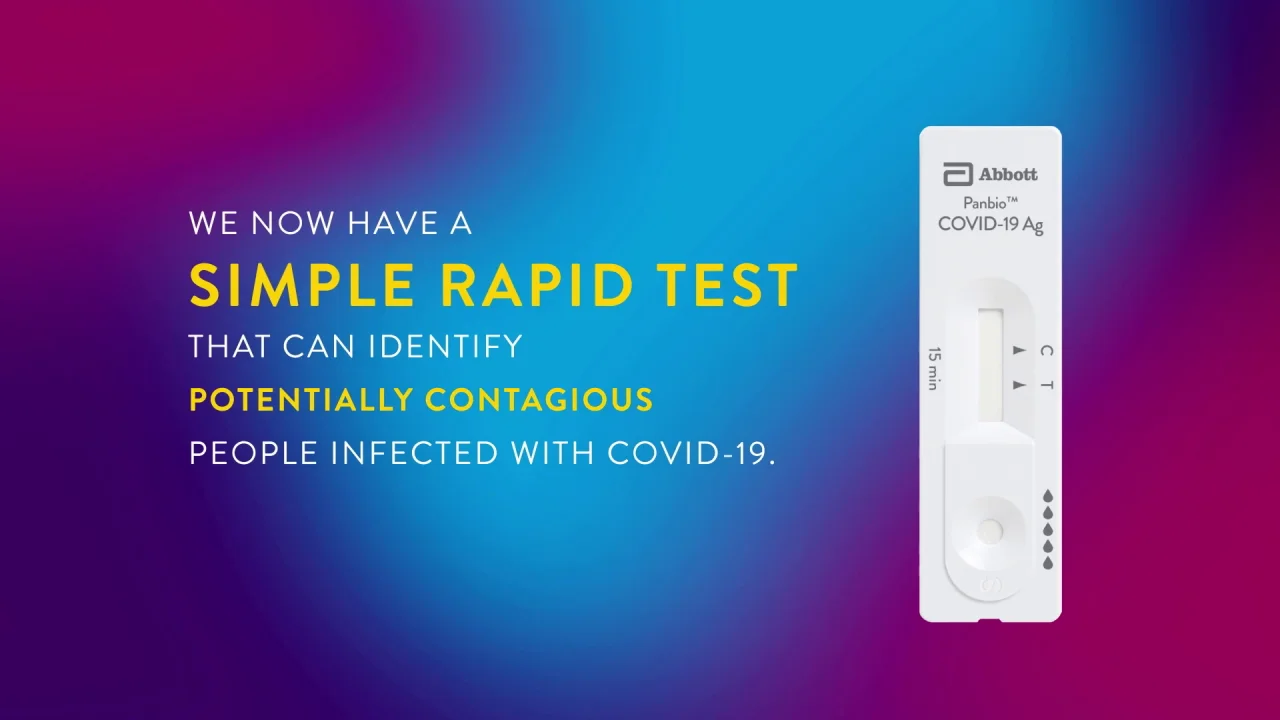
Panbio Covid 19 Ag Test Abbott Point Of Care

Abbott Labs Rapid 5 Covid 19 Test To Fill In Testing Gaps For Millions In The U S
Abbott S Point Of Care Covid 19 Test Detects Coronavirus In As Little As 5 Minutes Biospace

Panbio Covid 19 Ag Rapid Test Device Abbott Point Of Care

Point Of Care Testing Diagnostics Testing Newsroom

Laboratory Id Now Abbott Point Of Care Test Pcr Mobile Device Autodoc

Fda Authorizes Covid 19 Test That Doesn T Need Special Equipment Los Angeles Times

Abbott Id Now Covid 19 Instructions Modified
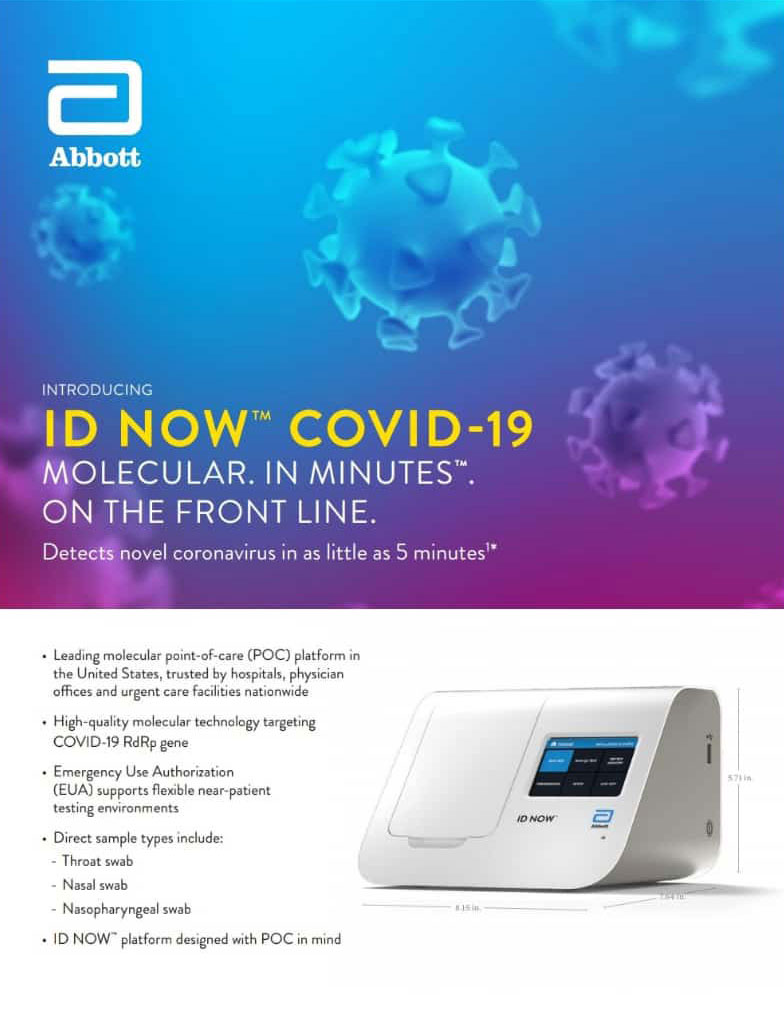
Laboratory Id Now Abbott Point Of Care Test Pcr Mobile Device Autodoc